Tutorials
Raw data
For this tutorial, we’ll download some low coverage 🦁 data for ten samples to illustrate the steps of quality control, trimming and mapping. While ENA allows using wget to download data (see and example here), for NCBI you need to use their tool sra-tools or parallel-fastq-dump. I’ve prepared an environment with an installation of parallel-fastq-dump, so we’ll use that here. Activate the environment first:
$conda activate parallel-fastq-dump
Now, create a folder to download the data to and use parallel-fastq-dump to grab accession numbers SRR10764405-SRR10764414 from NCBI:
$parallel-fastq-dump --sra-id SRR10764405 SRR10764406 SRR10764407 SRR10764408 SRR10764409 SRR10764410 SRR10764411 SRR10764412 SRR10764413 SRR10764414 --threads 4 --split-files --gzip
After you’ve downloaded the data, deactivate the environment:
conda deactivate
You should now have raw data for 10 individuals in your folder (paired-end, 20 files in total). Raw data come in .fastq.gz (or .fq.gz) format. Now let’s take a look at the data. Go to the folder where the data are stored, using cd, and use ls to display the contents of the folder. Most of the time, you’ll be working with paired-end data, meaning that each sample has two files. These are usually identified by _R1 and _R2, or _1 and _2. Those two files contain the forward and reverse reads, respectively. For more information about paired-end Illumina sequencing, watch this video.
Now let’s take a look at what’s inside these files. Type the following command:
zcat SRR10764405_1.fq.gz | less -S
The zcat command is used to unzip the file (note it ends with .gz), and less allows you to view it. Here we add -S to chop off long lines. Otherwise it wraps around and becomes messy. Try scrolling right and down, using the arrow keys. To quit less type q.
If you’d like to see the messy format, with long lines wrapping, try using head -n 20 instead of the less command. head shows the first part of the file, and -n 20 tells it to show the first 20 lines. If you’re really brave, try just using zcat, without less and see what happens. Press CTRL+C to escape the madness.
Now to the actual data. Each sequenced read is spread over four lines, one of which contains sequence and another the quality scores stored as ASCII characters. The other two lines are used as headers to store information about the read.
It’ll look something like this:
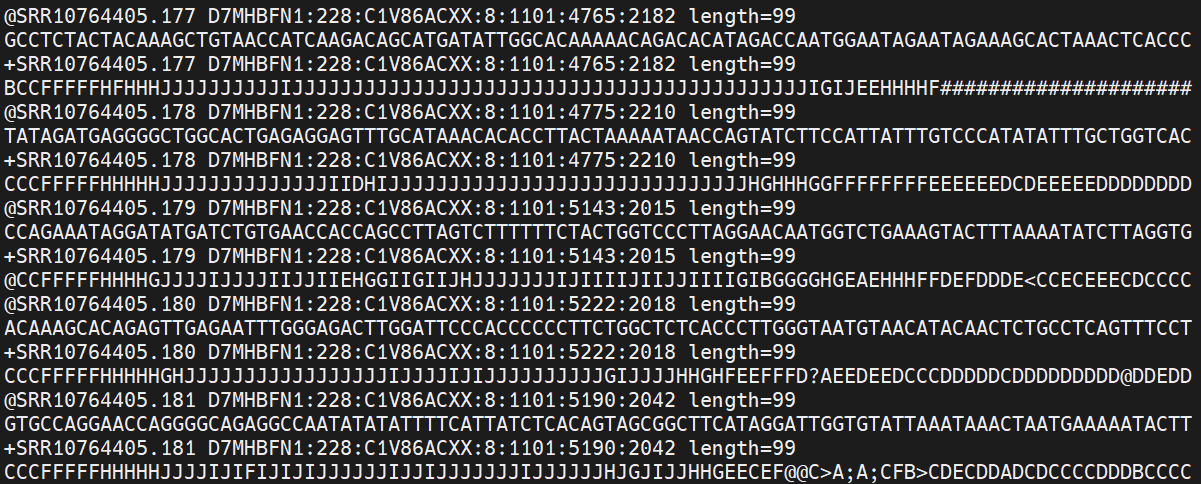
The first is the name of the read, with information about its location on the plate, or in this case the identifier from NCBI, where the data were downloaded from.
| Component | Meaning |
|———————|———————————————|
| SRR10764405.177 | SRA run ID + internal read index |
| D7MHBFN1 | Illumina sequencing instrument ID |
| 228 | Run number on that instrument |
| C1V86ACXX | Flowcell ID |
| 8 | Lane number |
| 1101 | Tile number within the lane |
| 4765:2182 | X:Y coordinates of the cluster on the tile |
| length=99 | Read length |
The second line contains the sequence data. The third line is unused (identified with +, often this line is empty, but here it repeats the header from the first line). And the fourth line is the quality scores for the base calls. The FASTQ wikipedia page has a good figure depicting the logic behind how quality scores are encoded.
FastQC for quality control
The first step is to inspect your raw data to estimate overall quality. Scrolling through the data with less is obviously not going to work for this. So, we’ll use a software called FastQC to help us visualize overall data quality for each of our files. You can then attempt to improve your dataset by identifying and removing samples with failed sequencing.
Let’s start the run now. First make an output folder, where you will store the results in. Use mkdir to do this. Then run the fastqc command, and direct it to your newly created output folder by using -o.
/softwares/FastQC/fastqc * -o output_files/
On the screen, you’ll see the progress of your FastQC run.
Note: we use , which is a wildcard. It means *any file in the directory. You can use wildcards also more specifically, for example
*.fq.gz, which means all fq.gz files. Or*_1*, which is all files with R1 anywhere in their name, which comes in handy if you only want to process forward reads, for example.
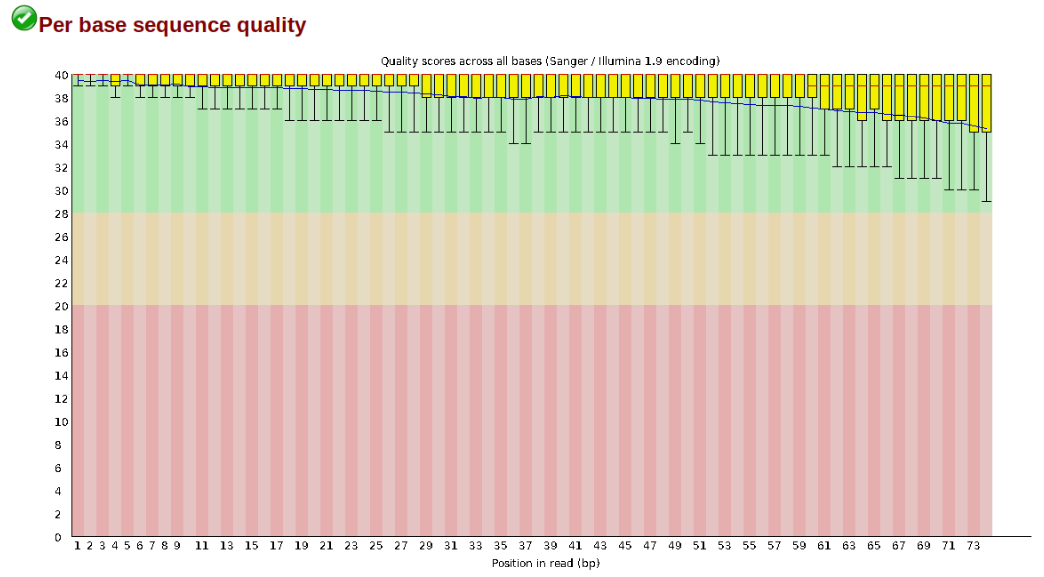
Another key QC procedure involves inspecting average quality scores per base position and trimming read edges, which is where low quality base-calls tend to accumulate. In this figure, the X-axis shows the position on the read in base-pairs and the Y-axis depicts information about Phred quality score per base for all reads, including median (center red line), IQR (yellow box), and 10%-90% (whiskers). As an example, here is a very clean base sequence quality report for a 75bp library. These reads have generally high quality across their entire length, with only a slight (barely worth mentioning) dip toward the end of the reads:
In contrast, here is a somewhat typical base sequence quality report for R1 of a 300bp paired-end Illumina run of another dataset:
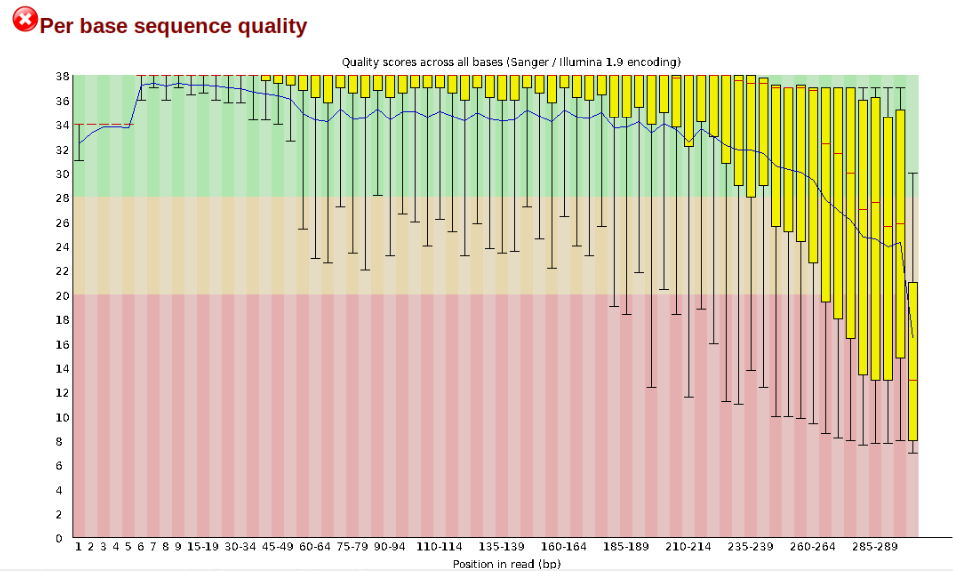
This figure depicts a common artifact of current Illumina chemistry, whereby quality scores per base drop off precipitously toward the ends of reads, with the effect being magnified for read lengths >150bp. The purpose of using FastQC to examine reads is to determine whether and how much to trim our reads to reduce sequencing error interfering with basecalling. In the above figure, as in most real dataset, we can see there is a tradeoff between throwing out data to increase overall quality by trimming for shorter length, and retaining data to increase value obtained from sequencing with the result of increasing noise toward the ends of reads.
When everything is done, navigate to your output folder using cd and see what files were created with ls.
You’ll see that for each input file, FastQC created an .html and a .zip file. You want to look into the html file, which contains all the information you need for now. If you’re using Mobaxterm, you can download the .html files from the panel on the left side on the screen. Or simply open it by right clicking and selecting “Open with…”, and then open it in Chrome or another browser. If you’re on Linux/Mac, you have to download the file. Open a new terminal window and navigate to where you want to download it to, e.g. your Downloads folder. Then use the following command:
scp user@cluster:~/output_files/*.html .
You’re now telling your computer to grab the files from the server. It will therefore ask you to provide your password again. The . at the end is the location the files will be downloaded to. It means here, the current directory, so if you’re in the Downloads folder, this is where your html files will end up in.
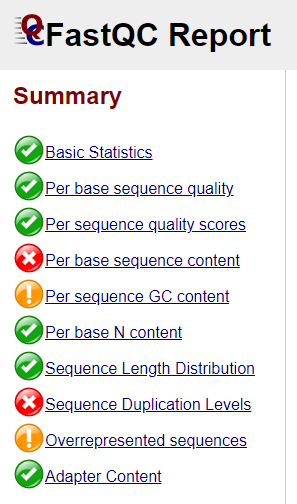
Open one of the html file in your browser. You’ll see plots like the one above. On the left side there is a summary of the results, highlighting those sections which require your attention. Is there anything in our data we should be concerned about?
Often, you’ll have a large number of samples, and you don’t want to waste time looking at tons of individual html files. MultiQC can help you summarize the results of multiple fastqc files. Unfortunately, MultiQC needs another python version than the one which is installed system-wide. We need to first create and activate an environment which allows MultiQC to run properly. Run the following lines:
conda config --set ssl_verify no
conda create -n multiqc --file /home/uramakri/laurabertola/Tutorial_WGS_HD/admin/multiqc_env.txt
conda activate multiqc
conda install -c bioconda multiqc
Now, navigating into your output folder with the FastQC results, and do:
multiqc .
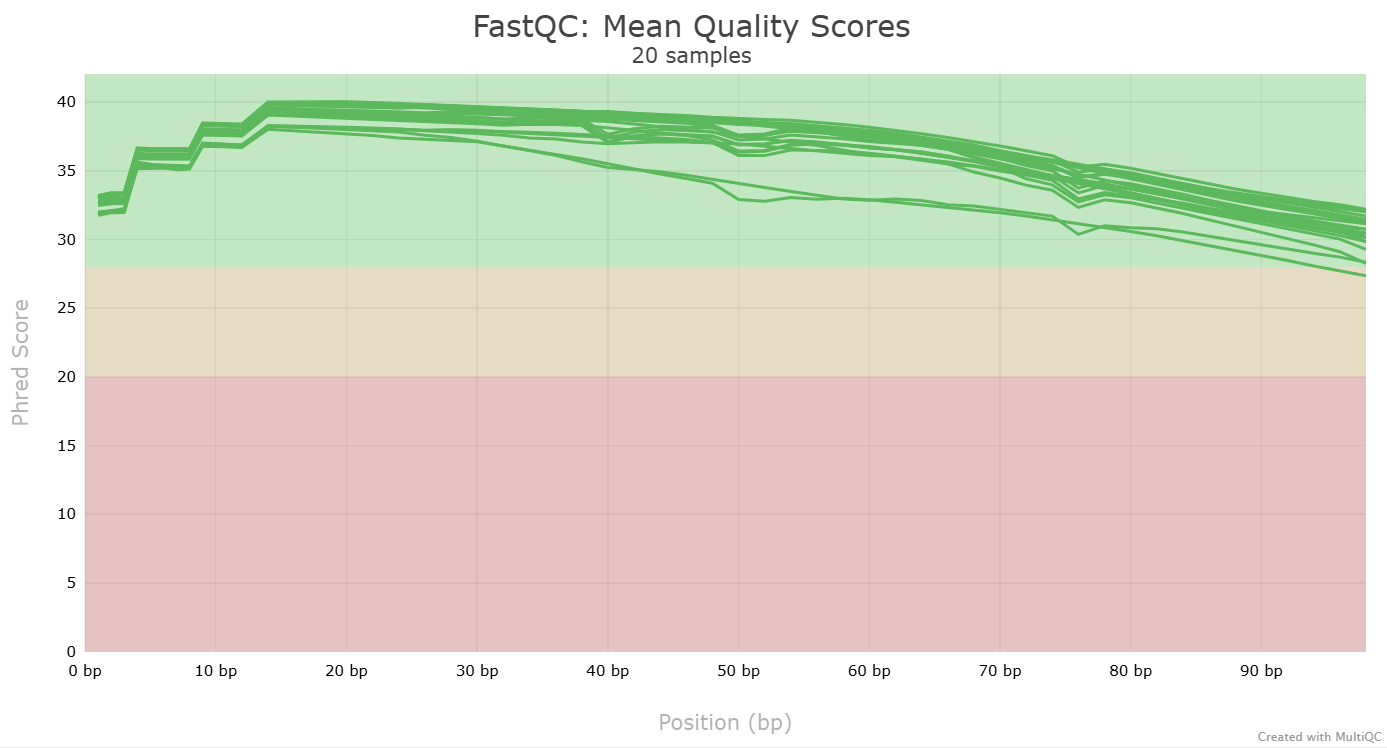
Look at the html file (download it to your computer, just like you did with the FastQC html files). It shows the results of all FastQC results in the same report. Note that the sample name appears when you hover with your cursor over a plot, so it is easy to identify which samples look strange and may require some more attention.
Let’s proceed to the next step, during which we can also tweak things if we’re not confident about the raw data quality. We no longer need the environment with the other python version, so first do:
conda deactivate
Trimming
There are multiple softwares to trim your data, e.g. Trimmomatic, cutadapt and TrimGalore! (which wraps around cutadapt and FastQC). We’ll use TrimGalore! here.
Run the following command:
for file in *_1.fq.gz; do
mate="${file/_1.fq.gz/_2.fq.gz}"
/softwares/TrimGalore/trim_galore --paired --illumina "$file" "$mate" --output_dir output_files
done
TrimGalore! does not like wildcards (*), so we have to loop over our files instead. For each file in the input_files directory which ends with _1.fq.gz, it finds the accompanying reverse reads file (mate=”${file/_1.fq.gz/_2.fq.gz}”), and it runs TrimGalore over both files. You tell it that there are –paired data, it should use –ilumina adapters for trimming (check the manual for more options, in case you’re using different adapters), and you also tell it to store the results in the –output_dir.
This is going to take a few minutes… ⌛
Trimming can be done in different ways. You can look for specific motifs (like adapter sequences), you can pick a quality cut-off to get rid of low quality bases at the ends of the reads, you can set the minimum read length to discard reads that become too short after trimming etc. So, there is a lot of flexibility depending on your needs. Note that by default TrimGalore! is discarding reads which end up as singletons, meaning if a read is being discarded its mate with automatically be discarded too. However, you can ask TrimGalore! to keep singletons by telling it –retain_unpaired. Also, if your first FastQC file showed a lot of adapter contamination, and you’d like to check how this has changed after running TrimGalore!, you can add –fastqc, so TrimGalore! will automatically run FastQC after trimming.
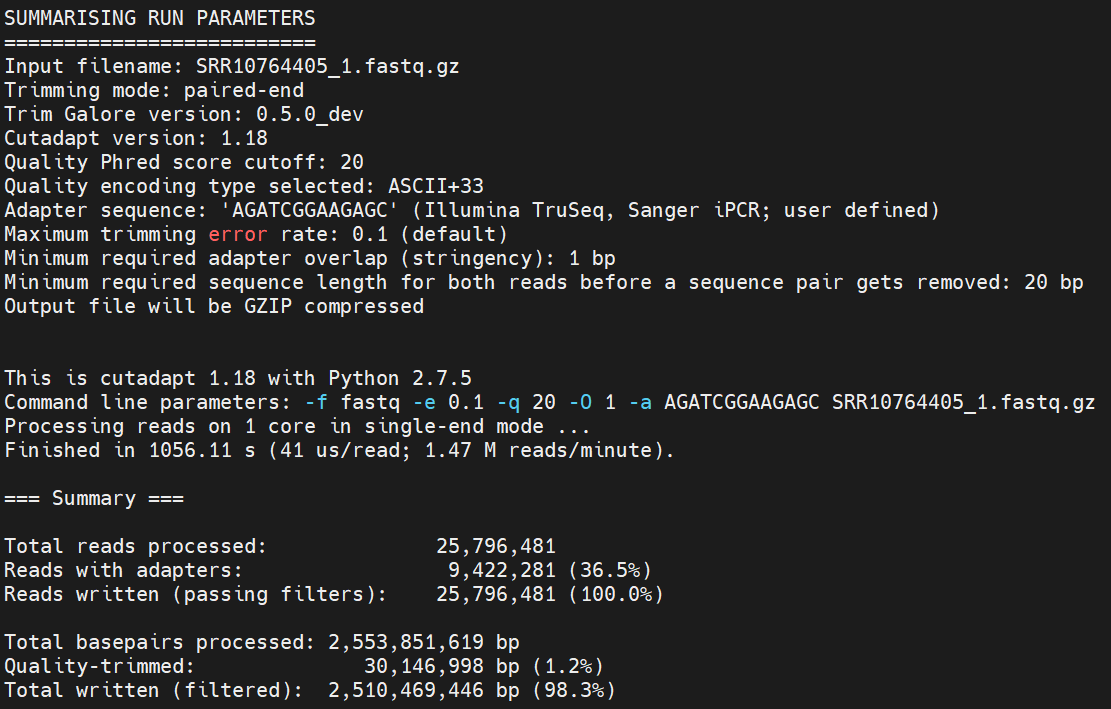
When the analysis finishes, look at the files it has created. You should have files with _val_1.fq.gz and _val_2.fq.gz. For each file, you also get a trimming report. Take a look what information is in there, using less.
If we’re happy with the quality of the reads that have made it through the trimming process, we’re ready to start the mapping!
Mapping
We’ll use this reference genome to map our data to. The reference files with all index files can be found in a folder called Reference/. Alternatively, you can download it yourself and index it, following the steps in Exercise 2 (just make sure that you download the right reference genome, and not the tiger genome which is being used in Exercise 2!).
You can also look into the reference file, the one ending with .fna, using less. You probably don’t want to scroll through an entire genome, so you can also look at the different scaffolds. They conveniently all start with a line starting with a >, so you can use grep to take a look at only those lines. Make sure you’re in the right folder when running this command.
grep ">" Panthera_leo_krugeri_HiC.fasta
For the mapping, we’ll use BWA MEM and a for loop to make it loop over all samples. The idea is the same as in the trimming step. We need to tell it which forward (_sub_1_val_1.fq.gz) and reverse (_sub_2_val_2.fq.gz) reads go together, tell it which reference genome to use, and where to store the output. The flag -t tells it how many cores it can use, so if you have a larger cluster and don’t have a lot of people running things simultaneously, you can probably request more cores. You should be in your Tutorials folder when running this command.
for file1 in output_files/*_sub_1_val_1.fq.gz; do
file2=${file1/_sub_1_val_1.fq.gz/_sub_2_val_2.fq.gz}
sample_name=$(basename "$file1" _sub_1_val_1.fq.gz)
bwa mem Reference/Panthera_leo_krugeri_HiC.fasta -t 2 "$file1" "$file2" > "output_files/${sample_name}_aligned_reads.sam"
done
This step will take some time… ⌛
When the mapping is finished, take a look at the files that were created using ls. Most downstream analyses use .bam files instead of .sam files. Also, most analyses like reads to be sorted by the order they occur on the genome, not by the order in which they were processed (which is the default .sam and .bam output). To convert .sam to .bam, and order the reads, do:
for file in output_files/*.sam; do
filename=$(basename "$file" .sam)
/softwares/samtools1.12/samtools view -S -b "$file" | /softwares/samtools1.12/samtools sort -o "output_files/${filename}_sorted.bam"
done
You’ll see that there are two commands, separated by a pipe (|). The first part tells samtools to extract (view) the .sam (-S) reads and convert them to a .bam (-b) format. Those data are then piped into the next command, where samtools is told to sort (-sort) the reads and save the result as an output file (-o).
Bam is a binary format, which is much faster to process. But because it is binary it is not human-readable anymore. If you’d like to see what it looks like anyway, take a peak into the .sam and .bam files using ‘less’. For the .sam file, you’re just seeing the header (the lines starting which @). If you’d like to see the actual alingment and don’t have the patience to scroll past the header, try this:
grep -v "^@" SRR10764405_aligned_reads.sam | less
We used grep before to only grab lines starting with >, here we use -v to tell it to only grab lines which do not start with @.
Next, we need to remove duplicates. Duplicates here refer to PCR replicates and optical replicates which falsely inflate the sequencing depth, affecting downstream variant calling. PCR duplicates are identical reads which result from the overamplification during PCR step of library preparation. Optical duplicates arise from sequencing machine artifacts, when a cluster is wrongly interpreted as multiple nearby clusters. We use Picard for this step. Run the following:
for file in output_files/*_sorted.bam; do
base=$(basename "$file" _sorted.bam)
java -jar /softwares/picard/build/libs/picard.jar MarkDuplicates \
I="$file" \
O="output_files/${base}_deduplicated.bam" \
M="output_files/${base}_duplication_metrics.txt" \
REMOVE_DUPLICATES=TRUE
done
We use a for loop again, and the command needs an input file (I), some information about the output file it should generate (O), as well as a metrics file (M). –REMOVE_DUPLICATES tells it to remove the duplicate reads, instead of just marking them. Check if the expected output files are generated, using ls.
We also have to index the .bam file again. This time we use samtools.
for bam in output_files/*_deduplicated.bam; do
/softwares/samtools1.12/samtools index "$bam";
done
Do ls to see what changed. You should now also have .bam.bai files.
We now have .bam files which are ready to go for downstream processing. But we haven’t really looked at the mapping quality yet. We can do so with the tool Qualimap. Run the following:
for bam in output_files/*_deduplicated.bam; do
sample=$(basename "$bam" .bam)
/softwares/qualimap_v2.2.1/qualimap bamqc \
-bam "$bam" \
-outdir output_files/qualimap_results/${sample}_qualimap \
-outformat HTML
done
It will create an bunch of files, and store them in a qualimap_results folder (note that you asked it to create this folder to put all the output files in). Download the entire folder (just like you did with the FastQC results) and look at the html file it in your browser. Because Qualimap does not embed the images in their html files (unlike FastQC), you need to download the entire folder. Take a look at the images at the bottom of the report (you can click the links on the right side of the page), especially the coverage across the reference and coverage histogram. It should look something like this:

The next step will be different from the variant filtering we’ve done for the high depth samples. Because these are low depth samples (~3X), we cannot call hard genotypes with certainty. Imagine you have a position with 2X or 3X coverage, how certain would you be this truly is homozogous if you only find 1 allele? So instead we’ll use genotype likelihoods as implemented in ANGSD. The next section deals with genotype-likelihood computation and probabilistic SNP filtering.
Housekeeping: we have created quite a few large files, and it is good practice to delete what you don’t need anymore. You could for example delete the .sam files, and the _sorted.bam files which were later deduplicated. You can remove files with
rm, but be careful! It will not ask you if you’re certain and it cannot be undone. Always keep the raw data, and keep the final .bam files as they will be needed for the variant calling.